1-9
Electrons can also lose energy as well as receive it. When an electron loses energy, it moves to a
lower shell. The lost energy, in some cases, appears as heat.
If a sufficient amount of energy is absorbed by an electron, it is possible for that electron to be
completely removed from the influence of the atom. This is called IONIZATION. When an atom loses
electrons or gains electrons in this process of electron exchange, it is said to be ionized. For ionization to
take place, there must be a transfer of energy that results in a change in the internal energy of the atom.
An atom having more than its normal amount of electrons acquires a negative charge, and is called a
NEGATIVE ION. The atom that gives up some of its normal electrons is left with fewer negative charges
than positive charges and is called a POSITIVE ION. Thus, we can define ionization as the process by
which an atom loses or gains electrons.
Up to this point in our discussion, we have spoken only of isolated atoms. When atoms are spaced
far enough apart, as in a gas, they have very little influence upon each other, and are very much like lone
atoms. But atoms within a solid have a marked effect upon each other. The forces that bind these atoms
together greatly modify the behavior of the other electrons. One consequence of this close proximity of
atoms is to cause the individual energy levels of an atom to break up and form bands of energy. Discrete
(separate and complete) energy levels still exist within these energy bands, but there are many more
energy levels than there were with the isolated atom. In some cases, energy levels will have disappeared.
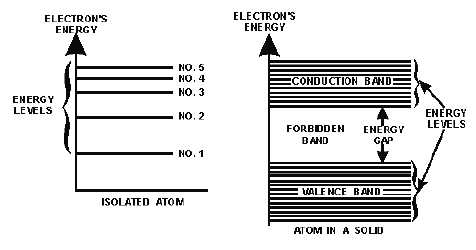
Figure 1-5 shows the difference in the energy arrangement between an isolated atom and the atom in a
solid. Notice that the isolated atom (such as in gas) has energy levels, whereas the atom in a solid has
energy levels grouped into ENERGY BANDS.
Figure 1-5.—The energy arrangement in atoms.
The upper band in the solid lines in figure 1-5 is called the CONDUCTION BAND because
electrons in this band are easily removed by the application of external electric fields. Materials that have
a large number of electrons in the conduction band act as good conductors of electricity.
Below the conduction band is the FORBIDDEN BAND or energy gap. Electrons are never found in
this band, but may travel back and forth through it, provided they do not come to rest in the band.
The last band or VALENCE BAND is composed of a series of energy levels containing valence
electrons. Electrons in this band are more tightly bound to the individual atom than the electrons in the
conduction band. However, the electrons in the valence band can still be moved to the conduction band
with the application of energy, usually thermal energy. There are more bands below the valence band, but
they are not important to the understanding of semiconductor theory and will not be discussed.