1-6
it into small particles; each of the particles still retains its original identifying properties of sugar. The
only thing that changed was the physical size of the sugar. If we continue this subdividing process by
grinding the sugar into a fine power, the results are the same. Even dissolving sugar in water does not
change its identifying properties, in spite of the fact that the particles of sugar are now too small to be
seen even with a microscope. Eventually, we end up with a quantity of sugar that cannot be further
divided without its ceasing to be sugar. This quantity is known as a molecule of sugar. If the molecule is
further divided, it is found to consist of three simpler kinds of matter: carbon, hydrogen, and oxygen.
These simpler forms are called elements. Therefore, since elements consist of atoms, then a molecule of
sugar is made up of atoms of carbon, hydrogen, and oxygen.
As we investigate the atom, we find that it is basically composed of electrons, protons, and neutrons.
Furthermore, the electrons, protons, and neutrons of one element are identical to those of any other
element. There are different kinds of elements because the number and the arrangement of electrons and
protons are different for each element.
The electron carries a small negative charge of electricity. The proton carries a positive charge of
electricity equal and opposite to the charge of the electron. Scientists have measured the mass and size of
the electron and proton, and they know how much charge each possesses. Both the electron and proton
have the same quantity of charge, although the mass of the proton is approximately 1,827 times that of the
electron. In some atoms there exists a neutral particle called a neutron. The neutron has a mass
approximately equal to that of a proton, but it has no electrical charge.
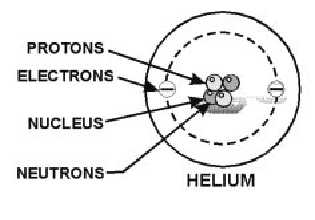
According to theory, the electrons, protons, and neutrons of the atoms are thought to be arranged in a
manner similar to a miniature solar system. Notice the helium atom in figure 1-2. Two protons and two
neutrons form the heavy nucleus with a positive charge around which two very light electrons revolve.
The path each electron takes around the nucleus is called an orbit. The electrons are continuously being
acted upon in their orbits by the force of attraction of the nucleus. To maintain an orbit around the
nucleus, the electrons travel at a speed that produces a counterforce equal to the attraction force of the
nucleus. Just as energy is required to move a space vehicle away from the earth, energy is also required to
move an electron away from the nucleus. Like a space vehicle, the electron is said to be at a higher energy
level when it travels a larger orbit. Scientific experiments have shown that the electron requires a certain
amount of energy to stay in orbit. This quantity is called the electron’s energy level. By virtue of just its
motion alone, the electron contains kinetic energy. Because of its position, it also contains potential
energy. The total energy contained by an electron (kinetic energy plus potential energy) is the main factor
that determines the radius of the electron’s orbit. For an electron to remain in this orbit, it must neither
gain nor lose energy.
Figure 1-2.—The composition of a simple helium atom.