1-8
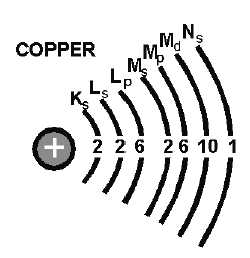
Figure 1-4.—Copper atom.
Valence is an atom’s ability to combine with other atoms. The number of electrons in the outermost
shell of an atom determines its valence. For this reason, the outer shell of an atom is called VALENCE
SHELL, and the electrons contained in this shell are called VALENCE ELECTRONS. The valence of an
atom determines its ability to gain or lose an electron, which in turn determines the chemical and
electrical properties of the atom. An atom that is lacking only one or two electrons from its outer shell
will easily gain electrons to complete its shell, but a large amount of energy is required to free any of its
electrons. An atom having a relatively small number of electrons in its outer shell in comparison to the
number of electrons required to fill the shell will easily lose these valence electrons. The valence shell
always refers to the outermost shell.
Q5. Define matter and list its three different states.
Q6. What is the smallest particle into which an element can be broken down and still retain all its
original properties?
Q7. What are the three particles that comprise an atom and state the type of charge they hold?
Q8. What is the outer shell of an atom called?
ENERGY BANDS
Now that you have become reacquainted with matter and energy, we will continue our discussion
with electron behavior.
As stated earlier, orbiting electrons contain energy and are confined to definite energy levels. The
various shells in an atom represent these levels. Therefore, to move an electron from a lower shell to a
higher shell a certain amount of energy is required. This energy can be in the form of electric fields, heat,
light, and even bombardment by other particles. Failure to provide enough energy to the electron, even if
the energy supplied is just short of the required amount, will cause it to remain at its present energy level.
Supplying more energy than is needed will only cause the electron to move to the next higher shell and
the remaining energy will be wasted. In simple terms, energy is required in definite units to move
electrons from one shell to the next higher shell. These units are called QUANTA (for example 1, 2, or 3
quanta).