1-7
The orbiting electrons do not follow random paths, instead they are confined to definite energy
levels. Visualize these levels as shells with each successive shell being spaced a greater distance from the
nucleus. The shells, and the number of electrons required to fill them, may be predicted by using Pauli’s
exclusion principle. Simply stated, this principle specifies that each shell will contain a maximum of 2n2
electrons, where n corresponds to the shell number starting with the one closest to the nucleus. By this
principle, the second shell, for example, would contain 2(2)
2
or 8 electrons when full.
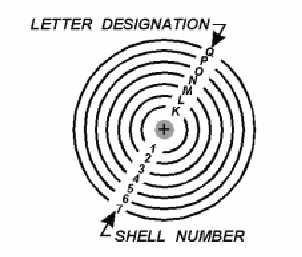
In addition to being numbered, the shells are also given letter designations starting with the shell
closest to the nucleus and progressing outward as shown in figure 1-3. The shells are considered to be
full, or complete, when they contain the following quantities of electrons: 2 in the K(1st) shell, 8 in the
L(2nd) shell, 18 in the M(3rd) shell, and so on, in accordance with the exclusion principle. Each of these
shells is a major shell and can be divided into subshells, of which there are four, labeled s, p, d, and f.
Like the major shells, the subshells are also limited as to the number of electrons they contain. Thus, the
"s" subshell is complete when it contains 2 electrons, the "p" subshell when it contains 6, the "d" subshell
when it contains 10, and the "f" subshell when it contains 14 electrons.
Figure 1-3.—Shell designation.
Inasmuch as the K shell can contain no more than 2 electrons, it must have only one subshell, the s
subshell. The M shell is composed of three subshells: s, p, and d. If the electrons in the s, p, and d
subshells are added together, their total is found to be 18, the exact number required to fill the M shell.
Notice the electron configuration of copper illustrated in figure 1-4. The copper atom contains 29
electrons, which completely fill the first three shells and subshells, leaving one electron in the "s" subshell
of the N shell. A list of all the other known elements, with the number of electrons in each atom, is
contained in the PERIODIC TABLE OF ELEMENTS. The periodic table of elements is included in
appendix 2.