1-12
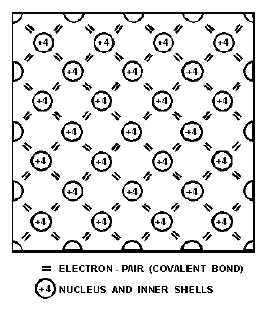
Figure 1-8.—A two-dimensional view of a silicon cubic lattice.
Q13. What determines the chemical activity of an atom?
Q14. What is the term used to describe the sharing of valence electrons between two or more atoms?
CONDUCTION PROCESS
As stated earlier, energy can be added to electrons by applying heat. When enough energy is
absorbed by the valence electrons, it is possible for them to break some of their covalent bonds. Once the
bonds are broken, the electrons move to the conduction band where they are capable of supporting
electric current. When a voltage is applied to a crystal containing these conduction band electrons, the
electrons move through the crystal toward the applied voltage. This movement of electrons in a
semiconductor is referred to as electron current flow.
There is still another type of current in a pure semiconductor. This current occurs when a covalent
bond is broken and a vacancy is left in the atom by the missing valence electron. This vacancy is
commonly referred to as a "hole." The hole is considered to have a positive charge because its atom is
deficient by one electron, which causes the protons to outnumber the electrons. As a result of this hole, a
chain reaction begins when a nearby electron breaks its own covalent bond to fill the hole, leaving another
hole. Then another electron breaks its bond to fill the previous hole, leaving still another hole. Each time
an electron in this process fills a hole, it enters into a covalent bond. Even though an electron has moved
from one covalent bond to another, the most important thing to remember is that the hole is also moving.
Therefore, since this process of conduction resembles the movement of holes rather than electrons, it is
termed hole flow (short for hole current flow or conduction by holes). Hole flow is very similar to
electron flow except that the holes move toward a negative potential and in an opposite direction to that of
the electron. Since hole flow results from the breaking of covalent bonds, which are at the valence band
level, the electrons associated with this type of conduction contain only valence band energy and must
remain in the valence band. However, the electrons associated with electron flow have conduction band
energy and can, therefore, move throughout the crystal. A good analogy of hole flow is the movement of
a hole through a tube filled with balls (figure 1-9).