1-35
The NEUTRON is a neutral particle in that it has no electrical charge. The mass of the neutron is
approximately equal to that of the proton.
An ELECTRON’S ENERGY LEVEL is the amount of energy required by an electron to stay in
orbit. Just by the electron’s motion alone, it has kinetic energy. The electron’s position in reference to the
nucleus gives it potential energy. An energy balance keeps the electron in orbit and as it gains or loses
energy, it assumes an orbit further from or closer to the center of the atom.
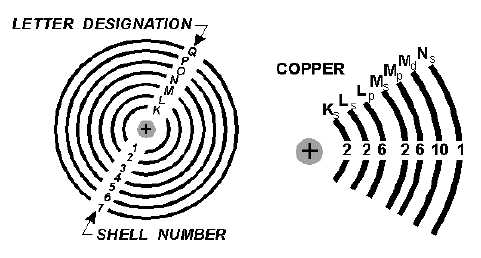
SHELLS and SUBSHELLS are the orbits of the electrons in an atom. Each shell can contain a
maximum number of electrons, which can be determined by the formula 2n
2. Shells are lettered K
through Q, starting with K, which is the closest to the nucleus. The shell can also be split into four
subshells labeled s, p, d, and f, which can contain 2, 6, 10, and 14 electrons, respectively.
VALENCE is the ability of an atom to combine with other atoms. The valence of an atom is
determined by the number of electrons in the atom’s outermost shell. This shell is referred to as the
VALENCE SHELL. The electrons in the outermost shell are called VALENCE ELECTRONS.
IONIZATION is the process by which an atom loses or gains electrons. An atom that loses some of
its electrons in the process becomes positively charged and is called a POSITIVE ION. An atom that has
an excess number of electrons is negatively charged and is called a NEGATIVE ION.
ENERGY BANDS are groups of energy levels that result from the close proximity of atoms in a
solid. The three most important energy bands are the CONDUCTION BAND, FORBIDDEN BAND, and
VALENCE BAND.