2-34
and the metal being joined. A solder joint is therefore chemical in nature rather than purely physical. The
bond is formed in part by chemical action and part by a physical bond.
The properties of a solder joint are different from those of the original solder. The solder is converted
to a new and different alloy through the solvent action. Two metals soldered together behave like one
solid metal. It is unlike two metals bolted, wired, or otherwise physically attached. These types of
connections are still two pieces of metal. They are not even in direct contact due to an insulating film of
oxide on the surfaces of the metals.
Temperature change does not affect the solder alloy. It withstands stress and strains without
damaging the joint. An unsoldered connection eventually becomes loosened by small movements caused
by temperature variations and by the gradual buildup of oxides on the metal surfaces.
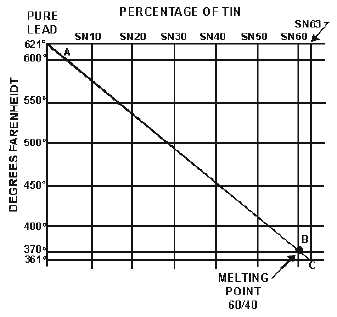
To understand fully the alloy or solvent action on molten solder, look at the tin-lead fusion diagram
shown in figure 2-35. This diagram shows that pure lead (point A) melts at 621º
F. Point C shows the
lowest melting point of the tin and lead alloy. The alloy at point C consists of 63-percent tin (SN63) and
37-percent lead. This is commonly called 63/37 solder. It has a melting point of 361º
F. This type of
solder, because of its very low melting point, is used in printed circuit boards and microminiature
electronic repair. As you can see from the chart, the melting point of the alloy is lowered when tin is
added to lead.
Figure 2-35.—Tin-lead fusion diagram.
The solder used to solder wires to electrical connectors, splices, and terminal lugs is a combination
of 60-percent tin to 40-percent lead (60/40 solder). The melting point of 60/40 solder is 370º
F, as shown
at point B of the figure. Type 60/40 solder is less expensive than 63/37 solder and is suitable for all
general uses.
Q37.
What two metals are used to from soft solder?
Q38.
Define the metal solvent action that takes place when copper conductors are soldered together.