2-35
Q39.
What is the tin-lead alloy percentage of solder used for electrical connectors, splices, and
terminal lugs?
FLUX
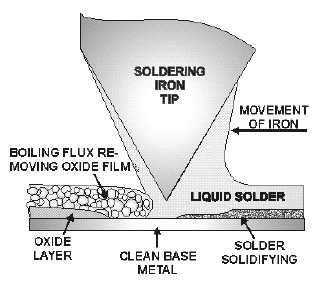
As you know, flux is a cleaning agent to remove oxidation during soldering. Heating a metal causes
rapid oxidation. Oxidation prevents solder from reacting chemically with a metal. Flux cleans the metal
by removing the oxide layer. This operation is shown in figure 2-36. As the iron is moved in the direction
shown, the boiling flux floats away the oxide film. The molten solder following the iron then fuses
rapidly with the clean surface of the metal.
Figure 2-36.—Action of flux.
There are two classes of flux: corrosive and noncorrosive. Zinc chloride, hydrochloric acid, and sal
ammoniac are corrosive fluxes. Corrosive flux should NEVER be used in electrical or electronic repair
work. Use only rosin fluxes. Any flux remaining in the joint corrodes the connection and creates a
defective circuit. Rosin is a noncorrosive flux and is available in paste, liquid, or powder form.
SOLVENTS
A solvent is used for cleaning and removing contaminants (oil, grease, dirt, and so forth) from the
soldered connection. Solvents must be nonconductive and noncorrosive. Solvents must be used in a
manner that keeps dissolved flux residue from "contact" surfaces, such as those in switches,
potentiometers, or connectors. Ethyl and isopropyl alcohol are acceptable solvents.
WARNING
These cleaning solvents are highly flammable and may give off toxic vapors.
Follow Navy safety precautions and take extreme care when using any flammable
solvent.
Q40.
What purpose does flux serve in the soldering process?
Q41.
What type of flux must be used in all electrical and electronic soldering?